Stem Cell Treatment Improves Autism Kids' Symptoms
Autism is a congenital mental disorder that has not yet been cured.
Although neuropathologic studies have not found consistent deficits in people with autism, scientists have long suspected that the disorder is linked to overall dysregulation of early fetal brain development.
Last month, The research team at Rutgers University published an article entitled "Autism NPCs from Both Idiopathic and CNV 16p11.2 Deletion Patients" in the prestigious scientific research journal Stem Cell Reports Exhibit Dysregulation of proliferation and Mitogenic Responses ".

In the past, many studies have used mice as a model. But when it comes to neurodevelopment in the brain, this form of research is limited because animals often don't show the same symptoms as human patients.
To mimic the early brain development of patients and find out what causes autism, the Rutgers team turned to stem cells that humans own.
They used induced pluripotent stem cells (iPSCs) technology to generate human brain stem cells that can accurately represent in vitro the changes experienced by autistic brains at an early stage of development, offering hope for effective treatments for the disorder.
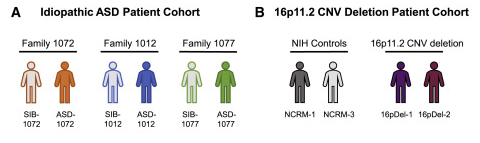
Ten subjects were included in the study, including three patients with idiopathic autism and three healthy siblings of the patients as controls, two patients with 16pDel (16P11.2 deletion syndrome) autism, and two healthy controls of the same sex. The ASD-1077 patient and the two 16pDel patients had macrocephaly.
The researchers induced stem cells from these subjects to differentiate into neural precursor cells (NPCS), a type of brain stem cell. Normally, neural precursor cells proliferate and differentiate into functional brain cells between 8 and 24 weeks of the 40-week gestation period in human fetuses.
Both the idiopathic and 16pDel autism cohorts showed dysregulation of neural progenitor cell proliferation compared with healthy controls.
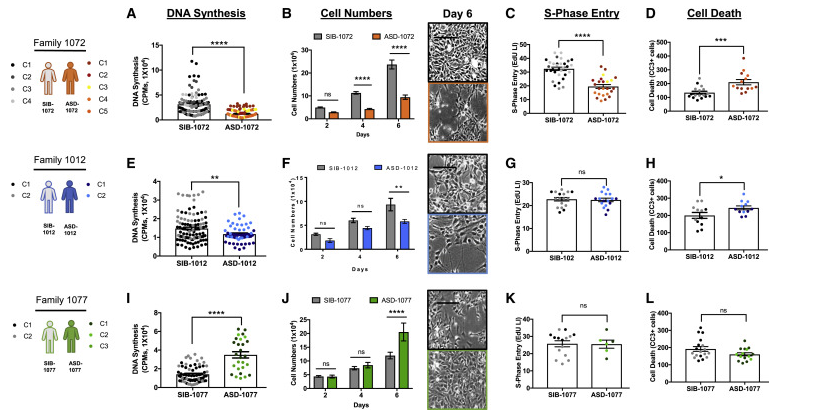 The NPCS of patients with ASD-1012 and ASD-1072 produced too few brain cells, even though they looked normal.
The NPCS of patients with ASD-1012 and ASD-1072 produced too few brain cells, even though they looked normal.
The large-headed ASD-1077 and the two 16pDel NPCS produced too many brain cells, which is the cause of the overgrowth in large-headed autism.
"The NPCS we studied from all [disease] samples showed abnormal proliferation, whether 'too little' or 'too much', suggesting that poor control of brainstem cell proliferation is an important basis for causality in autism," Emanuel Dicicco-Bloom, a professor of neuroscience and cell biology and pediatrics at Rutgers Robert Wood Johnson Medical School and an author of the paper.
Stem cells are human primitive cells, also known as "pluripotent cells", which can differentiate into various types of cells and tissues. They can not only be used as an in vitro model of autism, but also have a wide range of application prospects in regenerative medicine, drug development and precision medicine.
At present, stem cell transplantation technology has achieved initial success in the treatment of neurological diseases such as autism, proving that stem cells can improve and repair the structure and function of the nervous system to a certain extent.
According to a 2020 study by a Duke University research team published in Stem Cells Translational Medicine: Umbilical cord mesenchymal stem cells have shown safe efficacy in children with autism, and in this study, both single and repeated doses of infusion were effective.
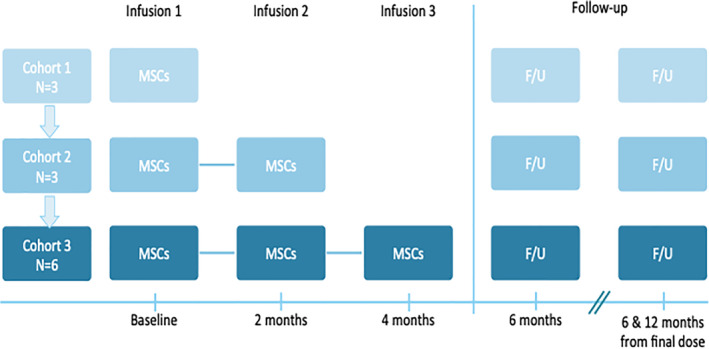
Researchers recruited 12 children with autism spectrum disorders, ranging in age from 4 to 9 years, and divided them into three groups: three patients in the first group received a single infusion, three patients in the second group received two infusions, and six patients in the third group received three infusions of equal doses spaced two months apart.
Among them, one child in the second group had mild hypotension and hypersensitivity reaction at the time of the second infusion, and the adverse reactions disappeared after symptomatic treatment, and then continued to complete the treatment without obvious discomfort. One child in the third group developed moderate hypotension, was treated without discomfort and completed treatment.
In general, umbilical cord mesenchymal stem cell infusion is safe and well tolerated in children with autism.
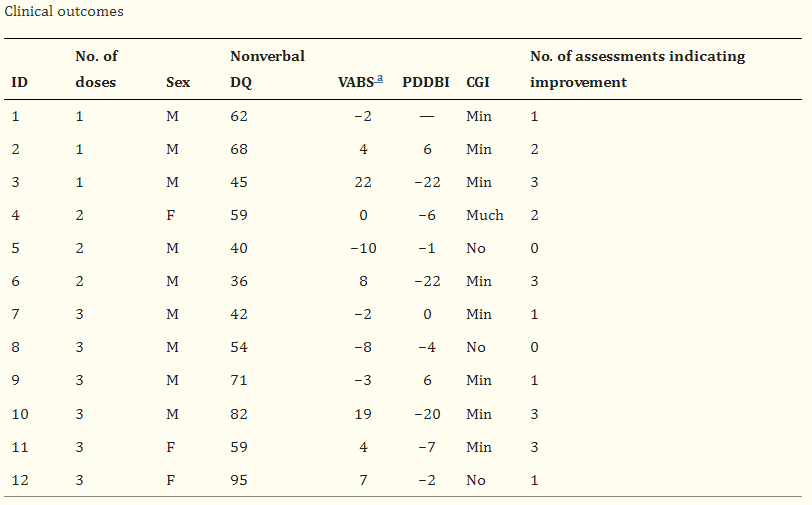
The researchers then evaluated the 12 participants, including physical examinations, clinical indicators and clinical psychometric scales, and found that a total of six patients showed improvement in the core symptoms of autism.
Two-thirds of the six PATIENTS SHOWED IMPROVEMENT in clinical measures, including two who received one dose of stem cell infusion, two who received two doses, and two who received three doses.
The study suggests that cell therapy can be useful in treating autism, but more research is needed to confirm its clinical application.
The Duke team recently launched a phase II randomized, double-blind study (NCT04089579) to evaluate the safety and efficacy of umbilical cord mesenchymal stem cells in improving social communication in children with autism. The study is expected to enroll 164 participants and complete in August 2023.
Let's hope that with advances in stem cell and regenerative medicine, people with autism will be able to benefit and live a new life!